Phage-Induced Heterogeneity in Microbes: Ecological and Biogeochemical Significance
Researchers at the Institute of Applied Ecology, Chinese Academy of Sciences (CAS), have unveiled how bacteriophages (viruses that infect bacteria) drive metabolic and functional variation among genetically identical microbes, reshaping our understanding of microbial ecology and its role in global biogeochemical cycles.
Microorganisms play vital roles in Earth’s ecosystems by driving essential processes such as carbon, nitrogen, and phosphorus cycling. Yet, even within genetically identical populations, individual cells can exhibit different behaviors, a phenomenon known as phenotypic heterogeneity. Until recently, the factors driving this variability were not well understood.
The research team, led by Dr. LIANG Xiaolong from the Innovation Group for Environmental Pollution Processes and Effects at the CAS Institute of Applied Ecology, synthesizes existing findings on phages (the most abundant biological entities on the planet) in driving this variability. Their paper, “Bacteriophage-driven microbial phenotypic heterogeneity: ecological and biogeochemical importance,” was published in npj Biofilms & Microbiomes.
The study explains that phages, depending on their life cycle strategy (lytic or lysogenic), can reprogram microbial metabolism in several ways, including regulating host gene expression, integrating or removing prophage elements, and expressing auxiliary metabolic genes (AMGs). These processes create distinct metabolic states among microbial cells, resulting in variations in physiological functions even within a single population.
At the community level, the researchers noted that phage-induced host phenotypic heterogeneity has a profound impact on microbial population dynamics and community structure. This variability shapes nutrient cycling and energy flow through processes such as the viral shunt, in which viral infection redirects organic matter from higher trophic levels back into the microbial loop. Such interactions play a significant role in the regulation and stabilization of carbon and nutrient cycles in marine and terrestrial ecosystems.
The researchers also review recent methodological advances that enable the study of these dynamics at the single-cell level. Advanced technologies, such as nanoscale secondary ion mass spectrometry (NanoSIMS), single-cell Raman spectroscopy, BONCAT-FISH, and microfluidic tracking, are now allowing scientists to trace the intricate phage-host interactions within individual cells.
Dr. LIANG’s team suggests that incorporating phage-driven microbial host phenotypic heterogeneity into ecological and carbon-cycle models could lead to more accurate predictions of ecosystem responses to global environmental change. Understanding these microscopic viral-microbial interactions is crucial, the researchers emphasized, for predicting how ecosystems store carbon and maintain stability under shifting climate conditions.
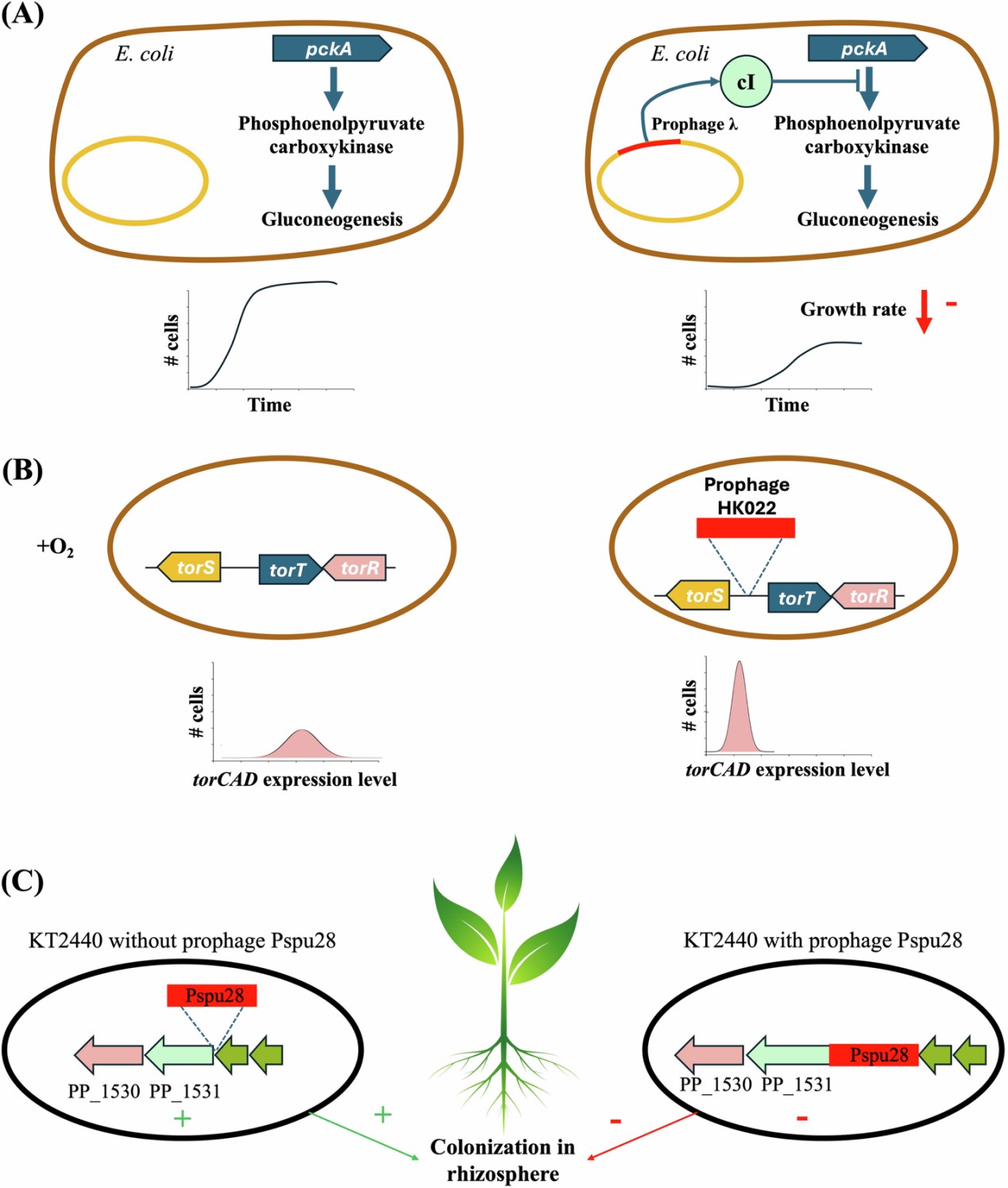
Figure 1. Schematic illustration of how phage infection induces phenotypic variation among microbial hosts (Image by LIANG Xiaolong).



