AMD with high concentrations of dissolved arsenic originated from mining of metallic sulfide ore deposits has been recognized as a major threat for water environment.
The efficient As removal from AMD is the focus of the current work. Few studies, however, have examined abio-oxidation of Fe(II) and/or As(III) in As-rich AMD for rapid As removal.
Recently, a research team led by Prof. JIA Yongfeng and WANG Shaofeng from Institute of Applied Ecology, Chinese Academy of Sciences elucidated fast efficient As removal process. Based on the effect of six actual AMD parameters on As removal efficiency, they determined As speciation and distribution in solution, and then analyzed the formation mechanisms of solid products.
The team found that pH has a major impact on As removal efficiency and oxidation. As pH increased from 2 to 7, the removal efficiency of As increased from 33% to 96% in 12 h. 12.4–45.4% of As(III) was oxidized to As(V) at pH 2–7 and the oxidation of As(III) to As(V) was most intensive at pH 5. Other five factors were less important to remove As.
Further observation showed that the process of Fe(III)-As(III+V) co-precipitate formation in the presence of Fe(II) and As(III) abio-oxidation had an optimum As removal efficiency within 6 h at pH 5, Fe/As of 2, oxygen flow rate of 1.6 L·min-1, 95 oC for AMD water containing As(III) concentration of 7.5 g·L-1.
These results revealed that the reaction mechanism in this work involved the oxidation of Fe(II) and As(III) by produced reactive oxygen species in Fenton-like reaction between Fe(II) and O2. Tooeleite/ferric arsenite hydroxysulfate, ferric arsenate and As(III+V)-bearing ferrihydrite act as the main products for As retention.
The paper, "Rapid Abiotic As Removal from As-rich Acid Mine Drainage: Effect of pH, Fe/As Molar Ratio, Oxygen, Temperature, Initial As Concentration and Neutralization Reagent ", was recently published online in Chemical Engineering Journal.
This study was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and Liaoning Province Doctoral Scientific Research Initiation Fund Project.
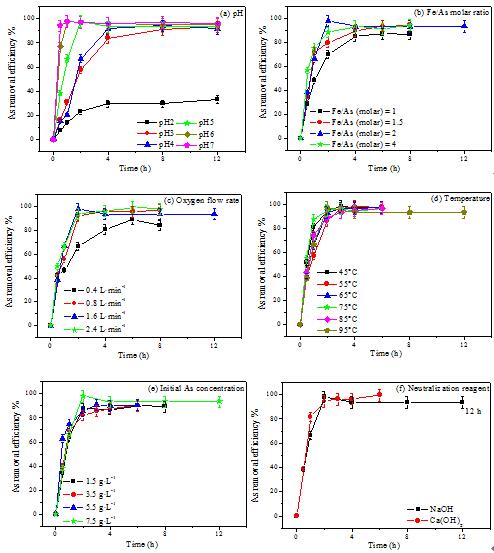
Fig1. A figure illustrating As removal efficiency as a function of time at various pH values (a), Fe/As molar ratios (b), oxygen flow rates (c), temperatures (d), initial As concentrations (e) and neutralization reagents (f) during the abio-oxidation treatment of Fe(II) and As(III) from As-rich AMD water (Image by WANG Shaofeng).
Email: yueqian@iae.ac.cn
Publication Name: WANG Shaofeng et al.




