Researchers Establish An Advanced Abio-combination Method for Rapid As Removal from As-Mn-rich AMD and Stable Final Solids
Acid mine drainage (AMD) with high levels of As(III) and Mn(II) is a worldwide environmental hazard.
Traditional microbial-mediated oxidation-precipitation processes cannot satisfy treatment demands of increasing As-Mn-rich AMD due to time-consuming microbial incubation and high-As inhibiting growth of microbes. The efficient abiotic As removal from AMD is regarded as the new direction.
In a new study published in Journal of Hazardous Materials on January 5, a research group led by Prof. JIA Yongfeng and WANG Shaofeng from Institute of Applied Ecology, Chinese Academy of Sciences has made great progress in an advanced abiotic oxidation-precipitation technology for fast efficient As removal from high-As(III)-Mn(II) AMD and low As-leaching solid waste.
The researchers found that As removal efficiency was strongly controlled by pH. Almost all of As removal occurred in 0.25–2h at pH 5–9. However, As removal efficiencies decreased to 99.4% at pH 8 and 92.6% at pH 9 after 8 h. As(III) oxidation efficiency was mainly affected by both pH and temperature, which increased from ~65% to ~99 as increasing pH from 5 to 9 and temperature from 25 oC to 95 oC.
Further, pH 8 and 95 oC were observed as the best parameters for both fast and efficient As removal together with more stable As-bearing solid waste.
The results showed that the mechanism involved produced reactive oxygen species (ROS) as oxidizers via Fenton-like reaction between Fe(II) and O2 for the formation of As-bearing Fe(III) (oxy) hydroxide and As(III) oxidation simultaneously accelerating catalytic Mn(II) oxidation on substrate Fe(III) (oxy) hydroxide surface to Mn(III, IV) oxides as successive oxidizers for further As oxidation. As-bearing six-line ferrihydrite was identified as the TCLP leaching stable solid product for As retention.
This study confirms As oxidation pathway on solid species and provides a reference for fast decrease of high-As pollution load and better storage of arsenic-bearing solid wastes.
The work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and Liaoning Province Doctoral Scientific Research Initiation Fund Project.
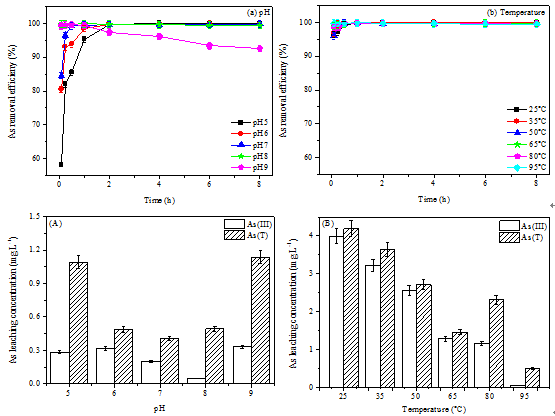
Fig 1. A figure illustrating As removal efficiency as a function of time at various pH values (a) and temperatures (b) together with leaching As concentrations in final solids by TCLP test as affected by pH (A) and temperature (B) during the oxygen-induced abio-oxidation of high-As(III)-Mn(II) AMD (Image by JIA Yongfeng's group)
Contact
YUE Qian
Institute of Applied Ecology, Chinese Academy of Sciences
Tel: 86-24-83970324
E-mail: yueqian@iae.ac.cn



